An Audacious Goal
By Liza Shevchuk

By Janet Wells.
When Herbert Wertheim School of Optometry & Vision Science professor Teresa Puthussery, OD, PhD, was a newly-minted clinician two decades ago, a patient in his early 20s showed up at the low vision clinic where she worked in her native Australia.
“We were the same age. He was planning a trip to Nepal to see the Himalayas and I had just returned from working in an eye hospital there,” she says. “He wanted to make the trip soon as he was progressively losing his vision from retinitis pigmentosa,” an incurable genetic disease that destroys the retinal photoreceptors, the light sensing cells of the eye.
Most patients at the clinic were elderly. But symptoms of retinitis pigmentosa (RP) usually start in childhood, leading to severe vision loss and blindness by age 40. “I thought, ‘What would it be like at my age to be going progressively blind, and there’s nothing we can do, not even any treatments in the next ten years?’” recalls Dr. Puthussery. “I wished I could say something hopeful about the outlook for his condition. I couldn’t. RP affects young people in the peak of their lives and we had no solutions.”
It was a crossroads moment, motivating Dr. Puthussery to return to school to study the biology and neurobiology of the eye, and fueling her ongoing quest for investigative and therapeutic breakthroughs.
Today, Dr. Puthussery would be able to have a different conversation with her Himalaya-bound patient, about a brighter future—one that she is helping to forge as part of a vision health “moonshot” funded through the prestigious Audacious Goals Initiative (AGI) for Regenerative Medicine.
The National Eye Institute (NEI) launched the AGI a decade ago with a prize competition that challenged scientists to imagine the greatest achievement for vision research during the next 10–15 years. The winner? Building a translational research pipeline to cure the most devastating and difficult-to-treat eye diseases through stem cell–derived regeneration of the retina.
Worldwide, about 285 million people are blind or visually impaired according to the NEI, a part of the National Institutes of Health (NIH). For many, vision loss results from degenerative retinal diseases such as RP, age-related macular degeneration (AMD), glaucoma, or diabetic retinopathy. If they’re available at all, current treatments for these diseases can only slow the process of degeneration. There are no cures.
The NEI’s audacious goal is to replace neurons of the retina that have been damaged by disease or injury and to restore their connections to the visual centers of the brain.
Dr. Puthussery, who studies the neural circuits of the retina and how they are altered by diseases such as RP and AMD, is part of a multidisciplinary AGI team recently awarded $3.7 million annually for five years. With Juliette McGregor, PhD, University of Roch-ester, and David Gamm, MD, PhD, University of Madison-Wisconsin, Dr. Puthussery is developing models that can gauge the survival and functional integration of transplanted light-sensing photoreceptors—the rods and cones—and retinal ganglion cells, which carry visual signals from the retina to the brain.
“I would never have imagined 20 years ago that this type of study would be possible. Back then we certainly didn’t have the scientific tools to approach the problem in this way,” Dr. Puthussery says. More recently, research efforts to understand and cure retinal diseases were hampered by the lack of tissue models that replicate the complexity of the human retina.
“Ten years ago, it wasn’t possible to grow a retina in a dish that was functional,” she says. “Now scientists have generated photoreceptor cells outside of the eye that can respond to light. I think in my lifetime we will see some of these vision restoration methods translated into the clinic. It’s already started.”
The Retinal Relay Team
While RP is relatively uncommon—about 100,000 Americans and 2.5 million people globally have the disease—AMD is the leading cause of vision loss for older adults, affecting nearly 200 million worldwide. People aged 55 and older are more likely to have AMD, when aging causes progressive damage to the macula—the part of the retina that mediates sharp, straight-ahead vision. AMD doesn’t cause complete blindness, but loss of central vision makes it harder to see faces, read, drive, or do close-up work like cooking or home maintenance.

Dr. Puthussery’s team is one of three that received AGI funding awards in fall 2021. Baked into the AGI model is having the three teams share data and technology and meet regularly with previous AGI-funded project teams, as well as an external scientific oversight committee to discuss progress and connect with technical resources.
“AGI is unique in that it brings these different groups and labs together to work toward a problem, instead of siloed-as-usual research. It’s a really good approach when you’re trying to solve a really complex problem like this one,” Dr. Puthussery says. “The open culture of sharing tools and tips really helps push the science forward.”
Dr. Puthussery’s team unites her expertise investigating retinal structure and function at the cellular level with stem cell biologists exploring retinal function in the living eye. Together they are leveraging imaging technology developed with prior AGI funding to locally ablate photoreceptors and evaluate restored retinal activity in vivo. Team leader Dr. McGregor, in collaboration with Dr. Gamm, transplants replacement photoreceptors from human-derived stem cells into damaged retinas using techniques designed to promote integration.
After transplantation, Dr. Puthussery’s lab receives tissue samples from her colleagues, and uses advanced high-resolution structural microscopy to evaluate the survival and integration of transplanted photoreceptors. In parallel studies, Dr Puthussery’s lab studies how retinal function is altered by photoreceptor degenerations to understand what factors could limit the efficacy of the cell transpant approach.
“The retina is an amazing model system,” she says. “It can be isolated from the eye and kept alive and light responsive under a microscope so we can make highly sensitive recordings from individual cells.”
Transplant tissue rejection was an early hurdle. “Of the large number of cells that you transplant, only a small fraction will survive. But there’s already evidence that at least some cells will survive in a host retina. Optimizing that is the challenge,” Dr. Puthussery says. “The next level is to ensure that photoreceptors not only survive, but that they can reach out and connect with the host retina.”
With AMD and RP the photoreceptors die, but the remaining neurons of the retina are still relatively intact, including the ganglion cells that send visual signals from the eye to the brain.
“If you can put new photoreceptors in and have them connect, they in principle could restore signaling to the brain and hopefully generate useful vision,” she says. But in retinas where photoreceptors cells have died, the rest of the retina is not completely normal. Understanding those changes is the team’s next challenge.
In a healthy retina, there’s a kind of relay race happening, Dr. Puthussery explains: “The photoceptors are first, detecting light stimuli before passing the baton on to the next runners, the bipolar cells. They pass the baton off to the ganglion cells, which send the signal from eye to brain.”
When photoreceptors die, however, what happens to the second set of runners? “What we know so far from preliminary data is that bipolar cells don’t die. They still have ‘arms,’ their arms are still outstretched, but they’ve lost the ‘glue’ in the tips of their dendrites to catch the baton,” she says. “This could be a real barrier to replacement therapy. We just don’t know yet. If the photoreceptors can hand off the baton, it’s possible bipolar cells will upregulate to produce the glue.”
Another unknown is whether the new photoreceptor cells can find the right bipolar cells—what Dr. Puthussery calls, “the ones on their relay team”—and be in the right place to connect. “The photoreceptors release a neurotransmitter, glutamate, but have to be close enough to the part of the bipolar cell that has a receptor for transmission.”
RP Research: A Personal Connection
In the Puthussery Lab, undergraduate research assistant Kristal Cosio conducts image analyses on mouse models of RP, studying the function of ganglion cells after photoreceptor degeneration. For her, the work is far more than a job. A Berkeley senior majoring in microbial biology, Cosio spent much of her life witnessing the progressive devastation of RP.
Her father, Jaime Cosio, noticed diminished night vision by the time he was a young teenager. But it wasn’t until he joined the army in the 1970s that his condition was diagnosed—due to his poor performance during night patrols. Instead of being shipped off to Vietnam, he was stationed at what is now Zuckerberg San Francisco General Hospital, working as a nursing assistant in the ear, nose, and throat clinic.
At Berkeley, where Jaime would go for eye exams, he was told there wasn’t anything that could be done for his condition. Cosio remembers her father losing a little more of his peripheral vision each year, his sightline gradually closing into a tunnel. By the time she was 12, Jaime was no longer able to drive or do many tasks at their home in El Cerrito, CA.
Raised solo by her father after her parents separated when she was a toddler, Cosio became his main caretaker. Once he was no longer able to work, she held down two jobs in high school. When Jaime passed away in 2015, his field of vision was at less than 10 percent. The family’s X-chromosome-linked RP has also led to greatly diminished vision for Cosio’s uncle, Arthur, who is now nearing age 70. (With a second X chromosome, Kristel has a genetic counterbalance against the disease.)
In summer 2021, Cosio attended a presentation by Dr. Puthussery as part of Berkeley’s NIH Bridges to Baccalaureate program, which offers exceptional transfer students from local community colleges a summer research fellowship.
“I emailed her right away that her research was personal to me,” recalls Cosio. “We talked about RP. She told me why she had decided to do this research, and we really connected on what it was like to be going through the stages of my dad losing his vision my whole life.”
Dr. Puthussery invited Cosio to join her lab in the 2021-2022 academic year, and she continued working there in the summer and now into her senior year—an experience that has steered her plans for a career in medicine in a new direction. Instead of using her training as an EMT and doula to specialize in labor, delivery, and women’s health, she’s hoping to follow in her mentor’s footsteps: a medical degree in ophthalmology and a PhD.
“I feel really lucky. Not only do I get to do research on RP, but I’m in lab with Teresa specifically,” Cosio says. “She’s walked me through every step of the research that I’ve been involved in and helped me become more comfortable and confident in wanting to pursue an MD-PhD. It’s made such a difference. I’d like to work directly with patients, but also be involved in the ways that we can change therapeutics.”
While Dr. Puthussery’s lab is located in the School of Optometry & Vision Science, she is well integrated into neuroscience exploration across campus. “We’re thrilled to have her state-of-the-art research program as part of the community,” says colleague Marla B. Feller, PhD, Paul Licht Distinguished Professor in Biological Sciences in the Division of Neurobiology, the Department of Molecular and Cell Biology, and the Helen Wills Neuroscience Institute.
Dr. Puthussery’s role in the AGI effort is just one example of her technical prowess and collaborative acumen, adds Dr. Feller. “She has built some incredibly exciting microscopes that are going to let her do experiments never done before that will generate a deep understanding of how to integrate new neurons into existing retinal circuits. These insights are fundamental for developing successful treatments for degenerative and inherited vision diseases. I do think her most exciting accomplishments are yet to come.”
As for making sweeping predictions for AGI breakthroughs, Dr. Puthussery is cautious—in the short term. But in five years? Her AGI team will know whether photoreceptors have the capacity to form functional connections, she says. In ten years, if the AGI effort continues to surmount barriers and push discovery, Dr. Puthussery anticipates retinal cell transplants moving to human clinical trials.
“It’s exciting that we are at a point, as a field, that we are ready to test this, and I’m very hopeful. But it is audacious. We don’t know whether this will work. It’s a long road. But that is part of the process,” Dr. Puthussery says. “It’s a huge deal to be involved in a project like this, a culmination of all my expertise to solve the most significant problem I can imagine.”
About the Photos
2. Dr. Puthussery working with her lab-mate.
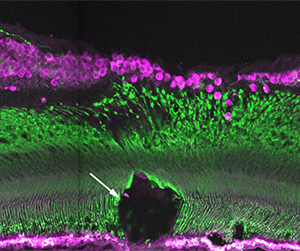
3. A retinal section showing a region of damaged foveal cones (green) in a model of age-related macular degeneration. This model system will be used to test cell-replacement therapies for photoreceptor degenerations.